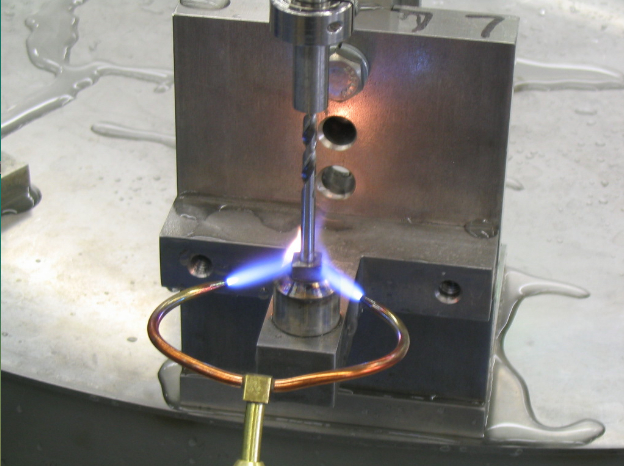
Brazing is a joining process whereby a non-ferrous filler metal alloy is heated to melting temperature (above 800°F) and distributed between two or more close-fitting parts by capillary attraction. At its liquidus temperature, the molten filler metal interacts with a thin layer of the base metal, cooling to form an exceptionally strong, sealed joint due to grain structure interaction. The brazed joint becomes a sandwich of different layers, each metallurgically linked to each other.
The brazing process can employ various heat sources such as torch, flame, acetylene, gas/air, induction, resistance, infrared, oven, and furnace. The process of brazing uses filler metals and alloys such as silver, copper, and zinc. Flux is necessary for brazing to remove and prevent reformulation of surface oxides on the base metals.
The brazing process produces strong, sealed, leak-proof joints. Brazing uses filler metals in solid form (such as rings and wire, slugs, washers, powder) as well as paste. Proper brazements begin with a good joint design. Brazing produces joints that meet specifications that meet mechanical performance, electrical conductivity, pressure tightness, corrosion resistance, and service temperature. High production, metal joining operations often employ brazing.
Learn more about Brazing at Fusion:
- Brazing Equipment
- Brazing Supplies
- Silver Brazing
- Brazing Alloys & Paste (Including Aluminum and Carbide. Or, learn how to apply Fusion brazing paste.)
- High & Low Temperature Alloys


USA: (440) 946-3300
EUROPE: +44 (0) 1279 443 122
Find Your Fusion Rep: USA | AMERICAS | EUROPE | ASIA-PACIFIC
Fusion Equipment in Action: Applications & Videos >>


